 2021-01-15
2021-01-15Respiratory syncytial (sin-SISH-uhl) virus, or RSV, is a?common respiratory virus that usually causes mild, cold-like symptoms. Most people recover in a week or two, but RSV can be serious, especially for infants and older adults. RSV is the most common cause of bronchiolitis (inflammation of the small airways in the lung) and pneumonia (infection of the lungs) in children younger than 5?year of age in the world.According to statistics, RSV infection is associated with more than 33 million cases of acute lower respiratory tract infections, and more than 110,000 deaths in children under 5 years of age worldwide each year. Among adults 65 and older, RSV kills about 7.2 per 100,000 people?a?year.?So far, early success has been achieved in the field of RSV vaccine development. Therefore, the detection of respiratory syncytial virus is very important.
The early symptoms of?COVID-19?are similar to those caused by viral infections such as flu?A, flu B, which will become epidemic in winter and spring. It is necessary for Combination of COVID-19, influenza viruses, respiratory syncytial virus molecular diagonostics.

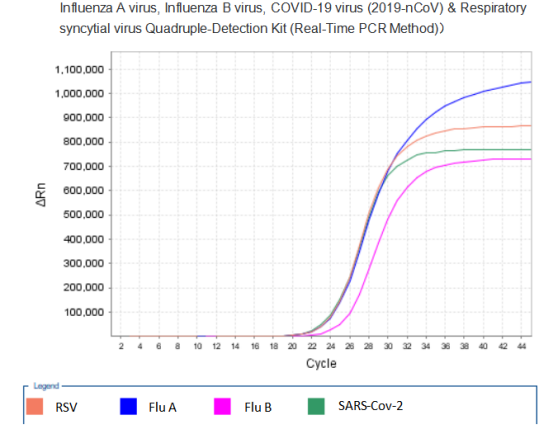
Recently, the Influenza A virus, Influenza B virus, COVID-19 virus (2019-nCoV) &?Respiratory syncytial virus Quadruple-Detection Kit (Real-Time PCR Method)?of Mabsky?Co., Ltd. has?been?approved by?CE. The Kit is used for the qualitative detection of COVID-19 virus (2019-nCoV), Influenza A, Influenza B virus?and Respiratory syncytial virus in nasal swab and throat swab specimens.?It?provides accurate, rapid and effective identification for the current respiratory infectious diseases.
PCR Amplification Curve
Mabsky CE Approved products:
COVID-19 Series | ||
No. |
Product name |
Spec. |
?1 |
COVID-19 virus(2019-nCoV)Dual-Detection Kit?(Real-Time PCR Method) |
25、50?T/Kit |
?2 |
COVID-19 virus(2019-nCoV)Triple-Detection Kit?(Real-Time PCR Method) |
25、50?T/Kit |
?3 |
Influenza A virus, Influenza B virus &?COVID-19?virus (2019-nCoV)?Triple-Detection Kit?(Real-Time PCR Method) |
25、50?T/Kit |
?4 |
Influenza A virus, Influenza B virus, COVID-19 virus (2019-nCoV) &?Respiratory syncytial virus Quadruple-Detection Kit (Real-Time PCR Method) |
25、50?T/Kit |
Devices & Consumables | ||
1 |
Automatic nucleic acid extractor |
MS-48/96 |
2 |
Real-Time PCR System |
SKY48/96 |
?3 |
The nucleic acid extraction kit |
50T/Kit |
?4 |
Nucleic Acid Extraction & Purification Kit(Magnetic bead method) |
32T/Kit; 64T/Kit |